Covaxin: ICMR & Bharat Biotech’s Landmark Collaboration | Patent, Royalties & Global Impact
Introduction
The development of Covaxin, India’s first indigenous COVID-19 vaccine, stands as a testament to scientific innovation and strategic collaboration. Beyond its medical significance, the partnership between the Indian Council of Medical Research (ICMR) and Bharat Biotech International Limited (BBIL) showcases the importance of intellectual property, government investment, and global outreach. This article delves into the financial, patent, and global distribution aspects of Covaxin, offering insights into its broader impact.
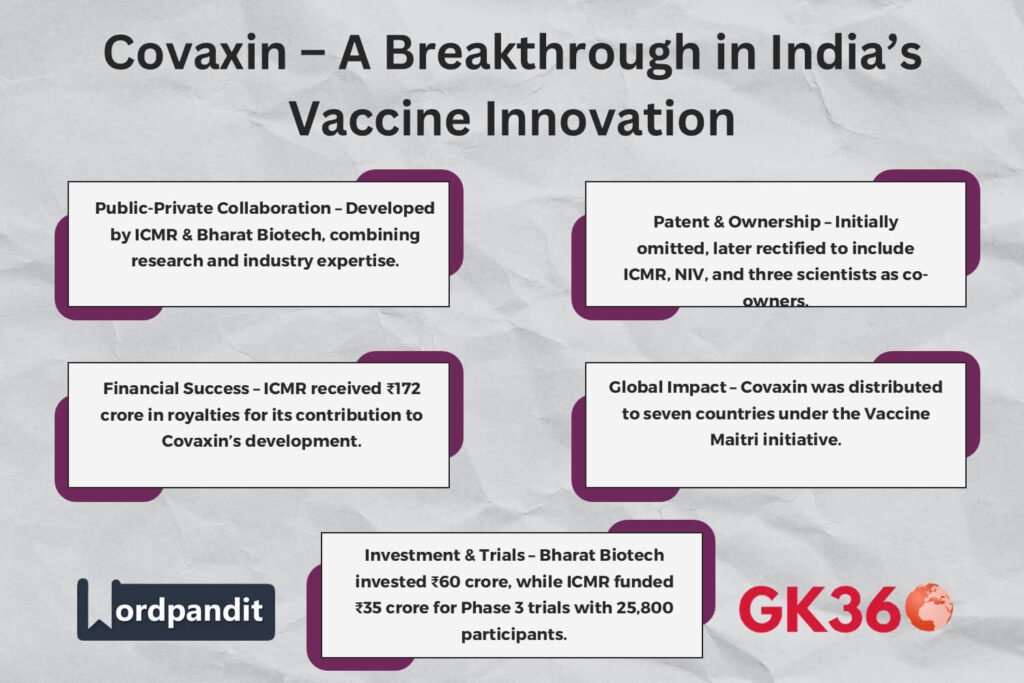
Table of Contents
- ICMR-Bharat Biotech Partnership
- Royalty & Financial Gains
- Patent & Ownership: The Rectification Issue
- Vaccine Maitri: India’s Global Outreach
- Phases of Development & Government Investment
- Lessons & Takeaways for Future Biotech Innovations
- FAQs
- Conclusion
ICMR-Bharat Biotech Partnership
The collaboration between ICMR and Bharat Biotech played a crucial role in Covaxin’s success. ICMR, through the National Institute of Virology (NIV), provided the isolated strain of the SARS-CoV-2 virus, while Bharat Biotech leveraged its expertise in vaccine development to manufacture and distribute Covaxin. This partnership bridged the gap between public sector research and private sector innovation, ensuring timely vaccine availability.
Royalty & Financial Gains
ICMR has received a significant royalty sum of ₹172 crore from Bharat Biotech for its contributions to Covaxin’s development.
Why Is This Important?
- Royalties are payments made to patent holders for the commercial use of their inventions.
- The financial success of Covaxin reinforces the value of public-private partnerships in scientific advancements.
- It highlights the role of intellectual property in funding future research and innovation.
Patent & Ownership: The Rectification Issue
Initial Oversight
At the outset, ICMR was not listed as a co-owner or co-inventor in the Covaxin patent application. This omission led to concerns over proper credit allocation and public sector stake in the vaccine’s intellectual property.
Government Intervention & Correction
- The oversight was rectified after government intervention, ensuring that ICMR and NIV were recognized as co-owners.
- Three key scientists from these institutions were also listed as co-inventors.
- This correction safeguards the interests of publicly funded research institutions and establishes a precedent for future collaborations.
Vaccine Maitri: India’s Global Outreach
Global Impact
India’s Vaccine Maitri initiative extended Covaxin’s reach beyond its borders, strengthening diplomatic ties and supporting global health.
Key Highlights:
- Covaxin was supplied to seven countries, reinforcing India’s commitment to global healthcare.
- This initiative positioned India as a key player in international vaccine distribution and pandemic response.
- It demonstrated the role of vaccine diplomacy in strengthening international relations.
Phases of Development & Government Investment
Phase 1: Virus Isolation & Early Research
- ICMR and NIV were instrumental in isolating the virus strain and conducting early studies.
- These foundational steps were critical for vaccine formulation.
Phase 2: Bharat Biotech’s Role
- Bharat Biotech took charge of vaccine development, investing ₹60 crore in research and trials.
- Extensive safety and efficacy tests were conducted during this stage.
Phase 3: Large-Scale Clinical Trials
- ICMR contributed ₹35 crore towards Phase 3 trials.
- The study included 25,800 participants across 25 clinical sites, ensuring comprehensive data collection.
Lessons & Takeaways for Future Biotech Innovations
Key Learnings:
- Public-Private Partnerships: Essential for accelerating medical breakthroughs.
- Intellectual Property Rights: Crucial for recognizing contributions and ensuring equitable benefits.
- Strategic Investment: Government funding plays a significant role in large-scale vaccine development.
- Global Distribution Strategies: Vaccine diplomacy strengthens a country’s global standing.
FAQs
1. Who owns the Covaxin patent?
ICMR, NIV, Bharat Biotech, and three key scientists are listed as co-owners and co-inventors.
2. How does vaccine royalty work?
Royalties are payments made to patent holders or co-inventors when their patented technology is commercially utilized. In this case, ICMR earned ₹172 crore in royalties from Bharat Biotech.
3. What is India’s Vaccine Maitri initiative?
A government-led program that distributed vaccines, including Covaxin, to multiple countries as part of India’s global healthcare commitment.
4. What were the costs of Covaxin’s development?
- Bharat Biotech invested ₹60 crore in research and production.
- ICMR funded ₹35 crore for Phase 3 trials.
5. How did ICMR contribute to Covaxin’s success?
ICMR provided the virus strain, funding, and research expertise, playing a crucial role in vaccine development and trials.
Conclusion
The ICMR-Bharat Biotech partnership in developing Covaxin is a landmark achievement in India’s scientific and healthcare landscape. Beyond its immediate impact in combating COVID-19, this collaboration set new benchmarks in public-private cooperation, intellectual property management, and global vaccine distribution. As India continues to advance in biotechnology, Covaxin remains a case study for innovation, investment, and international cooperation.
Key Takeaways Table
| Aspect | Details |
|---|---|
| Developers | ICMR, NIV, and Bharat Biotech |
| Patent Ownership | Initially omitted, later rectified to include ICMR, NIV & scientists |
| Royalty Earnings | ₹172 crore received by ICMR from Bharat Biotech |
| Investment Breakdown | ₹60 crore by Bharat Biotech, ₹35 crore by ICMR for Phase 3 trials |
| Clinical Trial Scale | 25,800 participants across 25 sites in India |
| Global Outreach | Distributed to 7 countries under Vaccine Maitri |
| Key Lesson | Public-private collaboration and IP management are essential for future innovations |
Related Terms:
- Covaxin Development India
- ICMR Bharat Biotech Partnership
- Covaxin Patent & Ownership
- Covaxin Royalties & Financial Impact
- Vaccine Maitri & Covaxin Exports
- Public-Private Collaboration in Biotech
- ICMR Covaxin Intellectual Property
- India’s Role in Global Vaccine Distribution
- Covaxin Investment & Trials
- Lessons from Covaxin’s Development






